|
The formation of NOx
|
|
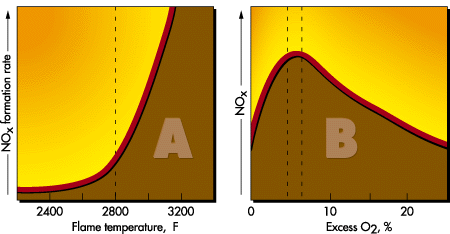
NOx emissions do not form in significant amounts until flame temperatures reach 2800 F. Once that threshold is passed, however, any further rise in temperature causes a rapid increase in the rate of NOx formation (A). NOx production is highest (B) at fuel-to-air combustion ratios of 5–7% O2 (25–45% excess air). Lower excess air levels starve the reaction for oxygen, and higher excess air levels drive down the flame temperature, slowing the rate of reaction. NOx reduction is the area of most concern today. Thermally
produced NOx is the largest contributor to these types of emissions. Thermal
NOx is produced during the combustion process when nitrogen and oxygen are
present at elevated temperatures. The two elements combine to form NO or NO2.
NOx is generated by many combustion processes other than boiler operation. It
combines with other pollutants in the atmosphere and creates O3, a
substance known as ground level ozone. NOx in boiler burners can be reduced with either pre-combustion
or post-combustion technology. Post-combustion technology allows NOx to form,
then breaks it down in the exhaust gases (a process called catalytic
reduction). This method is normally confined to larger, utility-size
equipment. Pre-combustion method prevents NOx from forming in the first
place. Pre-combustion NOx reduction is accomplished by either staging the
combustion process or recirculating flue gases into the combustion process
(FGR). FGR is accomplished by forcing the flue gases with a separate
fan back into the combustion zone (forced FGR), or by drawing the flue gases
through the combustion air fan (induced FGR). Both methods reduce the bulk
flame temperature in the furnace to inhibit the chemical reaction between the
nitrogen and oxygen. FGR systems reduce NOx emissions without reducing
efficiency. NOx values can drop to less than 20 ppm corrected to 3% O2
when burning natural gas. Uncontrolled NOx readings are generally in the area
of 80-120 ppm. |